Introduction
For food manufacturers, understanding and properly addressing non-conformance, process deviations, and quality incidents is fundamental to continuous improvement and risk mitigation. In this blog post, we will take a comprehensive look at these quality events, their causes and documentation processes – before delving deeper into how food businesses should assess the risk associated with non-conformities followed by corrective actions to be taken. We will discuss root cause analysis as a method for determining contributing factors before running through timescales for corrective and preventative actions in order to manage the allocation of responsibilities for measures taken towards consistent improvement and prevention of recurrence.
As we dive into the topic, you’ll be interested to know that Food Industry Hub offers integrated management systems for food manufacturers, which you can use to strengthen your assurance processes.
Table of Contents
Introduction: Defining Non-Conformance, Process Deviations, and Quality Incidents
Non-conformance, process deviations, and quality incidents refer to unintended or undesirable occurrences within the quality system. These can be expressed in many forms, including procedural non-compliance, recipe deviation, unwanted behaviours, pest activity, equipment failure, and many more.
Operational non-conformances can introduce risks to food safety, quality, authenticity, and legality – so it’s important to identify incidents and operational non-conformances when they occur, and take action to reduce the likelihood of repeat instances in the future.
It’s important to understand the distinction between a non-conformance, the consequence or harmful outcome; and the actions that need to be taken to remedy the situation. For example, if contamination is identified at the metal detector, the original non-conformance would be the contamination of product by metal (discovered through the use of foreign-body detection equipment), the consequences of that contamination incident could include product safety risks (were the contaminated product to be placed onto the market), and remedial actions might include disposal of the affected material and elimination of the contamination hazard (e.g. equipment wear/degradation).
To further illustrate, receipt of a customer complaint is not in itself a non-conformance or incident, but can serve as notification of a defect resulting from a quality incident.
Food Industry Hub Management Systems can significantly boost the effectiveness of your food safety and quality management system, leading to improved confidence and elevated quality assurance throughout your operations.
Clear and Detailed Documentation of The Non-Conformity
Clear and detailed documentation of any non-conformity that occurs is essential so that food manufacturers can review and understand problems that arise. Clearly, it would not be possible for the management team to collectively review unintended events in the absence of documented accounts of the incidents that have occurred.
For any such occurrence, it’s important to keep detailed and accurate records of the nature of the deviation and any apparent risks to product safety, quality, legality, and authenticity. Records should be sufficiently detailed to allow retrospective review of the incident, a timeline of events, affected materials and/or equipment, individuals involved, and any factors that may need to be trended of compared to similar incidents.
This includes documenting any changes or deviations from accepted procedures. It is important to save all relevant information regarding the issue, including notes on observations, corrective measures taken, and results achieved. Additionally, accurate records should be kept of all personnel involved in the process so that if necessary further investigation can be conducted. By having a clear and detailed record of any manifestations of non-conformance, manufacturers can quickly identify root causes and take swift corrective action accordingly.

Assessment of Consequences – Establishing Risk
Assessing the consequences of non-conformance, process deviations, and quality incidents is a critical step in managing risks as an outcome of an incident. This involves evaluating how severe the issue is and what potential risks it poses to safety or quality of end products. It also requires an understanding of any possible legal implications that may arise from these issues. Organisations should consider factors such as frequency, severity, impact on customers/consumers, cost involved in rectifying the problem etc. to get a comprehensive view of the risk associated with non-conformance.
All these assessments must be done thoroughly in order for food manufacturers to remain compliant while minimising any negative effects caused by issues that arise. The primary consideration should be the prevention of potentially unsafe product from being released onto the market, so the person in charge of assessing the consequences of a quality incident must have a thorough grounding in food safety. The assessment of consequences should not be limited to material directly affected by the incident, but should consider any residual risks that apply to the process or environment with the potential to impact subsequentially-processed materials. There is a very clear need to communicate risk information to the HACCP team for any food safety hazards identified.
Sign-up for the Food Industry Hub Mail Service
We regularly produce new content for food industry professionals, and the Food Industry Hub Mail Service is the best way to stay up to date with the latest additions.
Signup today to be added to the Food Industry Hub mailing list.
Immediate Corrective Actions
Immediate corrective actions serve to control the risks in the immediate aftermath of a non-conformance or quality incident. Typically, these are actions taken immediately as an incident is discovered, independent of the larger investigation and resultant corrective and preventative actions.
What was done to control the situation immediately as the deviation became apparent? How were risks mitigated?
Consider a glass breakage incident. The immediate corrective actions might include segregating the area, moving personal out in a controlled fashion, and replacing footwear to prevent spreading contamination. The immediate corrective actions serve to mitigate and control risks in advance of detailed investigation.

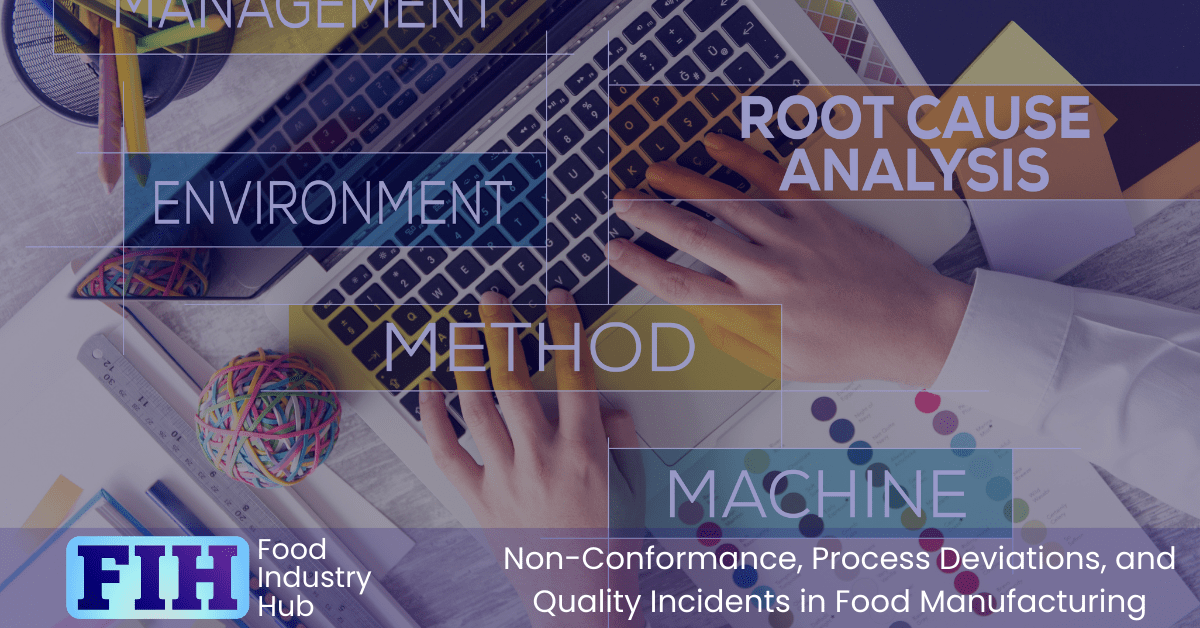
Root Cause Analysis – Determining Causes and Contributing Factors
Root cause analysis is a method used to identify and analyse the underlying reasons why certain issues have occurred. It involves an in-depth look into each contributing factor that has led up to the incident, such as processes, materials or people involved. This helps organisations better understand what went wrong, so they can make informed decisions on how to prevent similar problems from happening again in future.
By determining causes and contributing factors, food manufacturers are able to take actions designed to address the underlying causes with a view to prevention of recurrence. The intent is to address the factors that allowed for the occurrence of an incident, rather than focusing on the particular way a problem was manifested. This allows food manufacturers to identify any potential areas for improvement within their operations which can be addressed before further incidents occur. Root cause analysis is an important tool for food manufacturers that enables them to optimise quality assurance processes and reduce risks associated with non-conformance or process deviations.
The first step of root cause analysis involves gathering information related to the issue at hand – this could include records from observations made by personnel during production or data collected as part of the investigation. After all relevant information has been gathered it’s time for a thorough evaluation of each contributing factor that could have caused the problem; this includes looking into environmental factors, working practice, operational procedures, and people. Once all these aspects have been investigated the investigation should make an assessment of proximal causes and ultimately the root case.
Being able to accurately determine causes & contributing factors through root cause analysis provides food manufacturers with invaluable insight into their operations allowing them to improve process control and quality assurance measures.
Setting Appropriate Timescales for Corrective and Preventative Actions
It’s important that all necessary steps are taken in a timely manner so that problems can be dealt with efficiently and effectively. By setting realistic deadlines, food manufacturers will be able to ensure that any issues are addressed quickly before they become more serious or cause further contamination of products. This also allows them to identify potential areas for improvement within their operations which can then be addressed in order to reduce risk associated with non-conformance or process deviations.
When establishing appropriate timescales for corrective and preventative actions it’s important to consider variables such as complexity of issue at hand, resources available and overall impact on production if left unresolved. For instance, if the problem requires additional personnel or equipment then this should factor into how long an action might take – similarly, if the issue relates directly to a particular product batch, then immediate resolution may be needed due to potential health risks posed by contaminated goods. Having clear timelines set out helps keep teams focused while providing accountability in terms of who is responsible for taking each step towards rectifying the problem, which ultimately leads to better outcomes.
Allocating Responsibility – Managing Remedial Actions
It’s important for food manufacturers to clearly define who is responsible for each step of the process when allocating responsibility for managing remedial actions. This helps ensure that tasks are completed in a timely manner and allows organisations to have greater control over their corrective action plans as they can track progress from start to finish. It also provides accountability, ensuring that any issues are dealt with swiftly and efficiently without anything slipping through the cracks. Having clear roles assigned makes it easier for teams to work together collaboratively when dealing with complex problems or multiple investigations that run concurrently.
It’s important that responsibilities are allocated based on individual experience and expertise; this ensures that employees are able to effectively carry out their duties while minimising risk associated with non-conformance or process deviations. Additionally, assigning specific roles also helps prevent confusion which can occur when there is no clear understanding of who should be doing what – this ultimately leads to better outcomes as everyone involved can focus on their own area of responsibility instead of trying to do everything haphazardly. Allocating responsibility properly provides food manufacturers with an effective way of managing remedial actions while helping them achieve desired results more quickly and efficiently.
Effectiveness of Corrective and Preventative Actions – Verifying Improvement Initiatives
Verifying the effectiveness of corrective and preventative actions is helpful in verifying that food manufacturers are taking the necessary steps to improve their operations. This involves conducting audits and reviews to assess whether changes made have had a positive impact on product quality or process safety. It’s important for food manufacturers to regularly review existing procedures, processes, and standards in order to identify any areas where improvement can be made. This could include assessing elements such as risk management, employee training or equipment maintenance protocols. Additionally, it’s important for organisations to ensure that adequate measures are being taken when implementing new initiatives – which helps verify that objectives are being met and allows them to make any necessary adjustments should results not be satisfactory.
When verifying improvement initiatives, it’s important for food manufacturers to consider all aspects of the process from data collection & analysis through implementation & monitoring. This helps ensure that all relevant factors have been considered before making decisions about how best move forward with an initiative – as well as helping identify potential risks associated with changes which may need addressing first before proceeding further. Ultimately though, by verifying improvements through regular assessments & reviews, organisations can gain valuable insights into their operations while helping them achieve desired results more quickly and efficiently.
In Summary
Through careful management of non-conformities, process deviations, and quality incidents, you can ensure that your business remains compliant with regulations and legislation while minimising risk. It all starts with clear and detailed documentation of the issue, followed by an assessment of consequences to establish risk. By implementing immediate corrective actions in a timely manner, root cause analysis for determining underlying causes and contributing factors, setting appropriate timescales for corrective and preventative action initiatives, allocating responsibility for remedial measures as well as verifying improvement initiatives are crucial steps to manage non-conformance accurately. All these steps together will allow you to monitor the effectiveness of your remediation actions in order to protect your business from further issues.

Further Resources
Food Industry Hub serves the food industry with a range of digital resources for the benefit of both commercial food manufacturers and food industry professionals.
For food manufacturers, we offer integrated management systems that give every user a direct interface with your QMS.
For food industry professionals, we provide an extensive signposting service in addition to informational content we hope you’ll find useful as you face new professional challenges. We have very ambitious plans to expand the range of services offered, and currently present informational content on management, safety and quality, and professional success.















