Introduction
When it comes to food manufacturing, site standards are the foundation of food safety, quality, and legal compliance. Every aspect of your facility—from pest control to storage conditions and transportation—plays a crucial role in protecting products from contamination, deterioration, or security risks. A well-maintained site isn’t just about meeting audit expectations; it’s about safeguarding consumer trust and ensuring that every product leaving your facility is safe, authentic, and of the highest quality. Without robust site standards, even the most well-designed food safety systems can be undermined by poor environmental controls, inadequate maintenance, or overlooked risks in handling and transport.
This post takes a deep dive into the key requirements for site standards in food manufacturing facilities, offering practical insights into how each area contributes to overall food safety. From pest management and storage conditions to dispatch and transport, every detail matters when it comes to protecting your products and your business. Whether you’re refining your existing site standards or establishing them from the ground up, understanding these elements will help you build a facility that meets the highest industry expectations and operates with efficiency, security, and confidence.
As we dive into the topic, you’ll be interested to know that Food Industry Hub offers integrated management systems for food manufacturers, which you can use to strengthen your assurance processes.
Table of Contents
Key Takeaways
- Strong site standards are the foundation of food safety and quality, ensuring compliance with legal and industry requirements while protecting products from contamination and deterioration.
- A well-maintained facility supports operational efficiency, with structured processes for hygiene, storage, pest control, and transport reducing risks and improving product integrity.
- Regular monitoring and risk assessment are essential, allowing businesses to identify potential issues, implement corrective actions, and continuously improve site conditions.
- Staff awareness and engagement reinforce compliance, with proper training and accountability ensuring that standards are maintained in daily operations.
Food Industry Hub Management Systems can significantly boost the effectiveness of your food safety and quality management system, leading to improved confidence and elevated quality assurance throughout your operations.
External Standards and Site Security
Local Activities and Environmental Risks
When evaluating external standards, you’ll want to consider potential risks posed by the surrounding environment. Think about local businesses or neighbouring operations that could impact your site by releasing pollutants, dust, or effluent. Natural events, like flooding or strong winds, can also carry contaminants onto your site.
It’s not just about immediate threats—take a long-term view. If you’re in an area prone to environmental changes, whether from seasonal flooding or industrial developments, proactive risk assessments are essential to keeping your production facility safe and compliant.
Maintenance of External Areas
External areas under your site’s control need careful upkeep. Grassed or landscaped areas should be regularly tended and maintained to discourage pests and keep things tidy. Roadways and pathways should be well surfaced and in good repair, as potholes and uneven surfaces can accumulate debris and create contamination hazards.
An advisory: Make sure you review these spaces regularly and repair any damage as soon as possible. Neglected external spaces quickly become a liability.
Building Fabric and Pest Prevention
Adequate sealing is crucial for minimising contamination risks. Gaps around pipes, cracks in walls, and the presence of bird-roosting sites might seem minor, but they’re often gateways for pests and environmental contaminants. Sealing entry points and performing routine maintenance is critical to keeping unwanted visitors out.
A practical tip: regular inspections will help you catch issues early before they escalate into larger problems.
Site Security
You’ve got to think beyond physical barriers when it comes to site security. A visitor logging system is a great way to track who’s coming and going, and it’s key to ensuring that only authorised personnel enter sensitive areas like production and storage spaces.
Contractors and delivery drivers need to be briefed on site rules right from the start. And don’t underestimate the importance of training your staff on security awareness—it’s everyone’s responsibility, not just the security team’s.
An advisory: Assign a nominated person to oversee contractor activities in sensitive areas. That way, you maintain tight control without compromising operations.

Food Defence
When it comes to food defence, your focus should be on safeguarding your products, premises, and brand against malicious actions. The measures you implement must be robust enough to deter deliberate contamination or damage while ensuring compliance with legal and industry requirements. Here’s how you can approach this:
Understanding Food Defence Principles
If you’re involved in threat assessments and food defence planning, you need to have a solid understanding of both the site’s specific risks and the general principles of food defence. This includes recognising potential vulnerabilities across the site and understanding how these could be exploited. Where compliance requirements specify formal training for food defence, you must ensure it’s delivered and documented.
As a best practice, train the team responsible for food defence to look beyond the obvious. Encourage them to think creatively about potential threats, both internal and external, to ensure all risks are accounted for.
Conducting a Documented Threat Assessment
Your food defence strategy starts with a detailed risk assessment. This exercise should focus on identifying potential threats, both from within your organisation and external actors, that could deliberately harm your products or processes. Use the findings of this assessment to develop a comprehensive food defence plan tailored to your site.
This plan isn’t static; it needs to evolve with the business and the external environment. Review it at least annually, but also after significant events like the emergence of new threats or incidents that highlight vulnerabilities. Remember, your food defence plan must also align with legal requirements in the countries where your products are sold.
Managing High-Risk Materials and Processes
Raw materials and products identified as high-risk deserve special attention. Your food defence plan should outline specific controls to protect these materials from tampering or deliberate contamination. Prevention is your first line of defence, but where it’s not feasible, systems for early detection must be in place.
Monitoring these controls is just as important as implementing them. Ensure they’re reviewed regularly, with results documented to demonstrate compliance and identify areas for improvement.
Monitoring High-Risk Areas
For areas or processes identified as particularly vulnerable, you should define them clearly within your food defence plan. This could include intake points for raw materials or external storage facilities. Controls must be robust, with staff adequately trained in food defence procedures to mitigate any identified risks effectively.
Make sure the measures in place are practical and proportionate to the level of risk. Overcomplicating the process can lead to gaps in implementation, which could leave your business exposed.
By taking these steps, you can build a resilient food defence system that not only protects your products but also reassures your customers and stakeholders that you take food safety and security seriously.

Layout, Product Flow and Segregation
When it comes to your site’s layout, it’s not just about making things look orderly; it’s about safeguarding food safety, quality, and legality. A well-thought-out design can help you reduce contamination risks and streamline processes, making daily operations far more efficient and compliant.
Assessing Production Risk Zones
You’ll need to identify and assess the production risk zones for your products. This means recognising different contamination risks based on the type of processing carried out at the site. For instance, high-risk, high-care, and ambient high-care zones will each demand distinct control measures. Keep definitions clear and aligned with your food safety plan to ensure effective segregation.
Creating a Comprehensive Site Map
A map isn’t just a formality; it’s a strategic tool that brings clarity to operations and compliance. At the very least, your map should capture key elements like:
- Where high-risk and other product zones are located.
- Access points for personnel and raw materials.
- Routes for everything—from rework and waste to final products.
- Staff facilities like changing rooms, toilets, and smoking areas.
- Any necessary segregation for activities requiring specific time or space separation.
Having this visual reference helps you mitigate contamination risks while improving flow efficiency.
Managing Contractor and Visitor Access
Contractors and visitors aren’t always familiar with your site’s risks. That’s why it’s essential to ensure they know exactly where they’re going and what hazards may exist. Provide clear instructions to prevent accidental contamination—especially in sensitive zones.
Minimising Cross-Contamination Risks
The movement of personnel, raw materials, and waste can easily introduce contamination if poorly managed. Your process flows need to be meticulously planned, with controls in place to keep sensitive product areas protected. Demonstrating effective procedures for minimising risk will help you stay audit-ready and compliant.
Providing Adequate Working Space
Space constraints can lead to chaos—and potentially compromise food safety. Make sure your site allows enough room for all activities to be carried out properly without bottlenecks or overlap. Good spacing supports hygienic working conditions and reduces risks.
Designing Safe Temporary Structures
If you’re undergoing construction or refurbishment, temporary structures should be more than an afterthought. They need to be thoughtfully designed to avoid pest harbourage and maintain food safety standards. Ensure these structures don’t disrupt your operations or compromise product quality.
By paying attention to these layout, flow, and segregation requirements, you’ll build a site environment that not only meets compliance standards but also promotes safer, more efficient operations.

Sign-up for the Food Industry Hub Mail Service
We regularly produce new content for food industry professionals, and the Food Industry Hub Mail Service is the best way to stay up to date with the latest additions.
Signup today to be added to the Food Industry Hub mailing list.
Building Fabric, Raw Material-Handling, Preparation, Processing, Packing and Storage Areas
When it comes to your site’s building structure and facilities, it’s all about creating a safe, hygienic environment that supports food safety, quality, and efficiency. The design and maintenance of your spaces need to meet rigorous demands while ensuring processes can run smoothly without compromising product integrity.
One critical consideration is the prevention of dirt accumulation and condensation. You’ll want walls, ceilings, and floors that are not only durable but also easy to clean and maintain. For example, impervious floor surfaces that can withstand harsh cleaning chemicals are a must in many processing environments. Keep an eye on wear and tear—any cracks or damage can become havens for contaminants, so repairs should be timely and thorough.
Drainage is another essential factor. Poorly designed drainage systems can quickly lead to product contamination risks, especially if water doesn’t flow away efficiently. It’s a good idea to ensure wastewater is directed straight to drains wherever possible, avoiding pooling around process areas. Adequate drainage slopes can make a significant difference in reducing this risk.
Access points and elevated structures—such as walkways or mezzanine floors—need special attention. These should be carefully designed to prevent contamination from overhead exposure to open products or production lines. If your site uses walkways above open production areas, consider enclosing them or adopting contamination prevention measures to protect food safety.
Doors, windows, and ventilation systems must also play their part in maintaining a secure and sanitary environment. External doors should fit tightly to prevent pests from gaining access, and they shouldn’t remain open during production unless absolutely necessary. Adequate ventilation, meanwhile, is essential for controlling condensation and maintaining air quality.
Finally, lighting is often overlooked but plays a vital role in maintaining effective operations. Bright, well-positioned lighting allows for accurate product inspection and supports a clean and productive working environment. In poorly lit spaces, you risk missing contamination or defects that could compromise food safety.
Advisory: Regular inspections of structural elements like ceilings, ventilation systems, and drainage points can save you time and effort in the long run. Catching a small maintenance issue early on can prevent it from snowballing into a major problem that disrupts operations or leads to non-conformances during audits.

Utilities – Water, Ice, Air and Other Gases
Managing utilities like water, ice, air, and gases is essential for maintaining food safety and ensuring the integrity of your processes. Each of these elements has direct or indirect contact with products, making their monitoring and control essential to prevent contamination risks.
Water quality plays a foundational role. Whether it’s being used as a raw material, for cleaning, or in steam generation, the water must be potable at the point of use and fit for its intended purpose. Don’t underestimate the importance of regular water testing—microbiological and chemical analyses should be based on risk. This includes considering factors like the source of the water, how it’s stored, and past testing results. If you’ve got storage tanks, pay attention to how water is handled on-site to minimise stagnation and contamination risks.
A detailed and current schematic diagram of your water distribution system can be invaluable. It provides a clear picture of your water sources, treatment points, and recycling systems. This isn’t just helpful for audits but also essential for effective sampling and troubleshooting when anomalies are detected.
Air and gases, often overlooked, can be vectors for contamination if not properly managed. Compressed air used in direct contact with products should be filtered at the point of use to remove particles and contaminants. Likewise, if you’re using air or other gases as an ingredient, they must be monitored to ensure they meet food safety requirements.
Advisory Tip: Don’t wait until there’s an issue to review your utility systems. Conduct regular risk assessments, review schematic diagrams, and adjust your monitoring schedules based on any process changes or emerging risks. Ensuring robust filtration and maintenance schedules for air systems is just as critical as water management.

Equipment
The suitability, design, and management of production and product-handling equipment are crucial in maintaining food safety and ensuring efficient operations. Every piece of equipment should be purpose-fit to minimise the risk of contamination and support high hygiene standards.
When acquiring new equipment, a well-documented purchase specification is essential. This should detail legal requirements, hygiene considerations, and the specific operational purpose of the equipment. It’s a good practice to involve a multi-disciplinary team in this process, especially for complex or high-risk installations.
The design and construction of equipment should prioritise food safety. Smooth surfaces, impervious materials, and correct sealing techniques help prevent contamination risks. Ensure that equipment in direct contact with food complies with legal standards and supports effective cleaning.
Before commissioning new equipment, implement a risk-based commissioning procedure. This isn’t just about setting the equipment up—it’s a comprehensive process that ensures safe installation, operational readiness, and hygiene clearance. Inspections by qualified staff should verify readiness before use, with commissioning records forming part of your due diligence documentation.
Static equipment requires procedures for maintaining integrity and cleanliness over time, while mobile equipment presents unique challenges. Forklifts, scissor lifts, and other mobile units can introduce contamination if not effectively cleaned and maintained, particularly when moving between external and internal environments.
Advisory Tip: Keep a strict cleaning and maintenance schedule for all equipment, including rarely used items. Equipment left idle can accumulate debris and become a contamination risk if not properly cleaned and stored. For battery-operated equipment, avoid charging stations in open production areas.

Maintenance
A well-structured maintenance programme is essential for preventing equipment failures and reducing the risk of product contamination. Effective maintenance planning supports compliance, operational efficiency, and ensures the safety and legality of products.
Preventive maintenance should be central to your programme. By defining maintenance requirements during equipment commissioning and regularly reviewing these requirements, you can catch potential issues before they escalate into costly breakdowns. Remember to include all plant, processing, and mobile equipment in the schedule.
Damaged equipment introduces the risk of contamination by foreign bodies. Inspections should be conducted at defined intervals, with clear documentation of results and any corrective actions taken. Swift response to potential risks is critical for maintaining operational integrity.
Temporary repairs are sometimes unavoidable, but they should be managed carefully to ensure they do not jeopardise food safety or product legality. Document these repairs thoroughly and commit to permanent solutions within a defined timeframe.
Maintenance work presents its own contamination hazards. Implement hygiene clearance procedures and have authorised personnel inspect equipment to confirm the absence of contamination before resuming operations.
Note: Ensure all maintenance materials, such as lubricants, meet food-grade standards where there’s a possibility of direct or indirect contact with raw materials, packaging, or finished products. Keep engineering workshops clean and control the transfer of debris to production areas to minimise contamination risks.

Staff Facilities
Well-designed and maintained staff facilities are essential for safeguarding food safety and ensuring a hygienic environment for production. These facilities should be sufficient for accommodating all personnel while minimising the risk of product contamination.
Changing areas must provide safe and direct access to production zones without external contamination risks. If that’s not feasible, implement strict procedures and consider providing cleaning stations for footwear.
Proper storage facilities for personal items are essential, especially for those handling raw materials or working in preparation and packing areas. Ensure clear separation between clean production clothing and personal items or outdoor wear.
Hand-washing stations must be strategically located throughout production areas. These should offer essentials like soap, warm water, hands-free taps, and advisory signage to encourage hygiene best practices. Air driers or single-use towels should also be available to minimise contamination risks.
Toilets should not open directly into production areas and must be equipped with adequate washing facilities. Where toilet-based hand-washing stations are the only available option before re-entering production zones, ensure signage is in place to enforce hygiene compliance.
If smoking is allowed on-site, designate controlled areas that are isolated from production and storage zones, fitted with proper extraction systems, and managed to prevent product contamination.
Catering facilities, including vending machines, should be carefully controlled to avoid introducing food safety hazards, such as allergen risks. Ensure these areas operate under hygienic conditions.

Chemical Control: Keeping Risks at Bay
Managing chemicals in a food production environment requires a disciplined and thoughtful approach. Your goal should be to ensure that non-food chemicals never pose a threat to product safety or quality. Let’s walk through the key actions and considerations you need to take when implementing an effective chemical control system.
Approved Chemical Selection
Start by maintaining an approved list of chemicals for purchase. This ensures that only pre-vetted chemicals with known specifications and documented safety data sheets are brought onto site. Always verify that these chemicals are suitable for use in food production areas.
Proper Labelling and Identification
Mislabelled or poorly identified chemicals can lead to serious safety risks. All chemical containers should be clearly labelled and easy to identify at all times. This simple but essential step prevents accidental misuse and helps staff navigate their duties safely.
Secure Storage
Segregation is key. Designate secure storage areas for non-food chemicals, keeping them physically separate from raw materials, packaging, and processing zones. Access should be restricted to authorised personnel only.
Avoid storing strongly scented products near production areas—these can taint food products even when securely packaged.
Training and Usage
It’s essential that only trained personnel handle non-food chemicals. Training sessions should cover everything from safe handling procedures to managing spills and disposing of empty containers correctly.
Don’t forget to have a clear, documented plan for dealing with chemical spills. Prompt, proper response reduces risk to both employees and products.
Disposal of Chemicals
Outdated or obsolete chemicals are a risk waiting to happen. Ensure you have procedures in place to safely and legally dispose of or return these materials. Keeping the chemical inventory fresh reduces potential contamination risks and keeps your operation lean.
Handling Taint Risks
If you’re working on a task involving strongly scented or taint-forming materials, perhaps during building maintenance, ensure there’s a procedure in place to prevent taint contamination. This might involve scheduling work during production downtimes or installing temporary barriers to keep odours out of production zones.
By proactively managing the chemicals on your site, you’ll significantly reduce contamination risks and ensure a safer, more controlled production environment.

Metal Control: Minimising Foreign Body Risks
Keeping sharp metal implements and foreign-body hazards in check is critical to ensuring food safety and protecting your products from contamination. Here’s how you can approach metal control thoughtfully and practically.
Managing Sharp Implements
If your site uses knives, cutting blades, needles, or wires, it’s essential to have a documented policy outlining their controlled use and storage.
Consider these key actions:
- Tracking and Inspection: Maintain a record of inspections for each tool, noting their condition and checking for any signs of damage.
- Loss Investigation: If a sharp item goes missing, don’t treat it lightly. A thorough investigation should be conducted immediately to locate and account for the item.
- Prohibited Equipment: Avoid using snap-off blade knives entirely. These present an unacceptable contamination risk because broken fragments can end up in products.
By formalising these processes and training your staff, you can significantly reduce the likelihood of foreign body incidents.
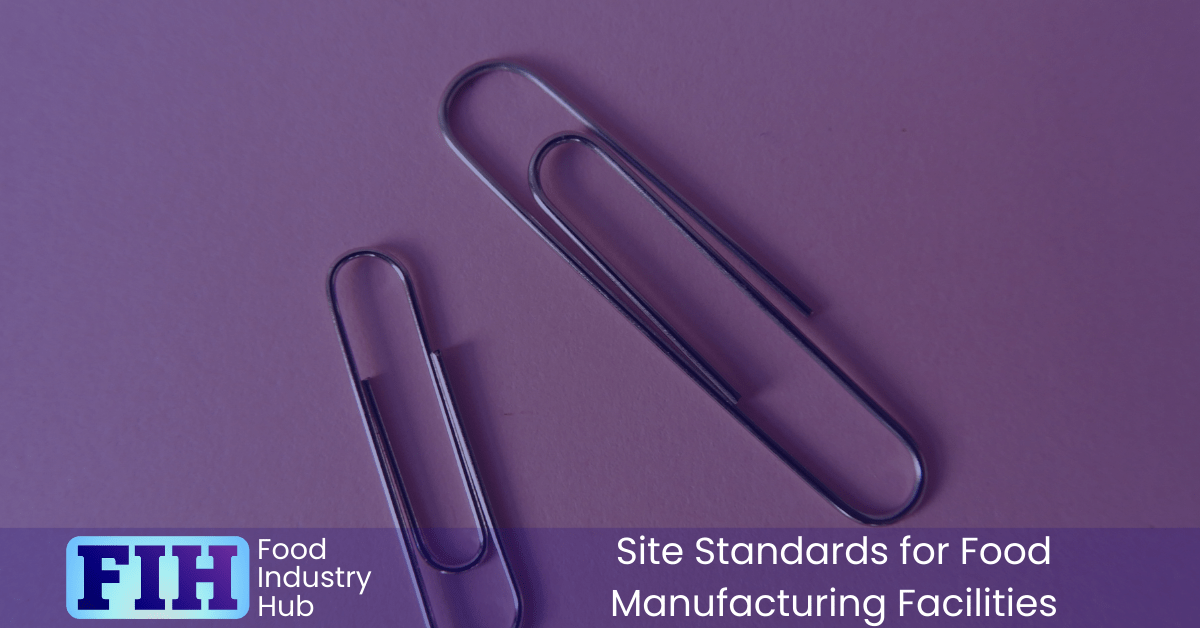
Packaging Hazards and Foreign Bodies
One often overlooked risk comes from packaging materials that introduce foreign objects like staples or small metal items. Managing this requires forward-thinking procurement policies and clear operational controls.
- Avoid Staples in Packaging: Sourcing packaging that uses staples or other similar hazards should be avoided wherever possible.
- Ban in Open Areas: Paper clips, drawing pins, and similar fasteners should never be used in open product zones.
- Control Measures: If items like staples are unavoidable in packaging, implement robust precautions to protect against contamination, such as visual inspections or magnetic separation systems.
Being vigilant about metal control throughout your operations supports a culture where product integrity and food safety are front of mind, fostering both regulatory compliance and customer trust.

Managing Risks from Glass, Brittle Plastic, Ceramics, and Similar Materials
When you’re working in food manufacturing, controlling the risk of contamination from materials like glass and brittle plastic is essential. Even a small fragment can cause significant product safety issues, potentially leading to recalls or damaging customer trust. That’s why robust procedures for managing these risks are non-negotiable.
To start, you need to ensure that glass and other brittle materials are either completely excluded from open product areas or suitably protected against breakage. This might sound straightforward, but it requires meticulous attention to detail. Think about windows, light fixtures, or even brittle tools and equipment. They all need to be shielded or controlled to reduce the chance of contamination.
Having a well-documented procedure for managing these materials is key. This should cover everything from keeping a detailed inventory of all glass and brittle items to regular condition checks. It’s not just about knowing what’s there — it’s about proactively monitoring their condition to spot potential risks before they become issues. Replacing items promptly when damage is detected is also crucial.
Of course, even with precautions in place, accidents can happen. When they do, you’ll need a clear and efficient response plan. Breakages must be handled in a way that minimises disruption and ensures no risk to food safety. This means training your team so they know exactly what to do, including isolating any affected products, thoroughly cleaning the area, and carefully inspecting equipment before resuming operations. Don’t forget to document every breakage incident and safely dispose of any contaminated product — comprehensive records can be invaluable during audits.
Glass windows present another unique challenge. If there’s any risk of breakage, they need to be reinforced or protected. Light fittings are also worth careful consideration, particularly bulbs and strip lights around production lines. Wherever possible, these should be shielded with protective covers. When covers aren’t practical, alternative measures like wire-mesh screens or regular inspections can help detect potential contamination.
By paying close attention to how brittle materials are managed, you can maintain a safer production environment, reduce contamination risks, and ensure you meet both regulatory and customer expectations.

Products Packed into Glass or Brittle Plastic Containers
For products packed into glass or other brittle containers, maintaining robust controls is essential for safeguarding both food safety and production efficiency. To begin with, you need to segregate the storage of these containers from raw materials, products, or other types of packaging. This separation reduces the risk of cross-contamination or accidental damage, which could lead to dangerous glass contamination in your products.
In the unfortunate event of container breakages, your approach should be swift, methodical, and comprehensive. Have clearly documented instructions that cover everything from removing and disposing of any at-risk products near the breakage point to thoroughly cleaning affected areas. Be mindful that cleaning methods must prevent further dispersal of fragments—high-pressure water or air, for example, should be avoided. Instead, opt for specialised cleaning tools that are colour-coded and stored separately to avoid confusion or misuse.
Waste containment is equally crucial. Use dedicated, clearly labelled, and accessible lidded containers for collecting damaged containers and fragments. After cleaning, inspect the production equipment carefully to ensure that all contaminants have been effectively removed. Grant production restart authorisation only once you are confident that there’s no risk of further contamination. Keeping the area clear of broken glass at all times is not just best practice—it’s an essential component of maintaining safe operations.
Maintaining accurate records of container breakages is another vital step. Even when no incidents occur during a production period, make sure that’s documented as well. Reviewing these records can help you spot patterns or recurring issues, offering valuable insights into how you might improve your processes or container handling procedures.
By integrating these practices into your food safety and quality management system, you not only ensure compliance but also demonstrate a strong commitment to product safety and operational excellence.

Wood Controls
When it comes to using wood in food manufacturing environments, strict control is essential to protect food safety. Generally, wood should be avoided in open product areas due to the inherent risk of contamination from splinters, damage, or degradation. However, there are exceptions where wood is a necessary part of the process, such as the maturation of certain products where contact with wood imparts desirable characteristics.
If using wood cannot be avoided, you need to implement a risk-based monitoring system to assess its condition regularly. This ensures that the wood remains in good condition, free from splinters or damage that could compromise product safety. By conducting these checks at appropriate intervals, based on the level of risk, you can mitigate potential hazards and maintain control.
For any wood that comes into direct contact with food, it must be fit for purpose. This means ensuring it is completely free from splinters, taints, or contaminants. If wood treatments are necessary, they should always comply with relevant legislation and be specifically approved for food use.
A proactive approach to monitoring and maintaining wood materials not only ensures compliance but also demonstrates your commitment to safeguarding product integrity. By adhering to these standards, you can balance process needs with rigorous safety measures.

Other Physical Contaminants
Preventing physical contamination from raw material packaging is essential during processes such as debagging and deboxing. Implementing specific procedures at these stages helps to protect the integrity of the materials being introduced into production areas. Proper techniques for removing packaging and carefully disposing of it are fundamental to reducing the risk of physical contaminants entering the product stream.
When it comes to handheld equipment, including stationery items, mobile phones, and tablets, these must be tightly controlled in open product areas. Unauthorised items can be a significant source of contamination, especially if they contain small detachable components or foreign materials. One approach is to limit use to site-issued equipment, which can be specifically chosen or modified to minimise risks. For example, pens used in critical areas might be designed to be easily detectable by foreign body detection systems, or you might limit their use to designated zones where contamination risks are easier to manage.
Foreign Body Detection and Removal Equipment
Minimising the risk of product contamination through effective foreign-body detection and removal requires thoughtful planning and implementation. To start, a documented assessment should be conducted for each production process as part of your food safety plan. This helps identify where foreign-body contamination risks exist and the most suitable detection or removal equipment to address them. Options may include filters, sieves, metal detectors, X-ray devices, magnets, and optical sorting equipment.
Defining the type, location, and sensitivity of foreign-body detection equipment forms a critical part of your site’s control measures. Factors such as the nature of your raw materials, ingredients, and final products must be considered to ensure the equipment performs effectively. Industry best practice often suggests carefully validating these systems to ensure they are fit for purpose and provide reliable protection against contamination.
Testing the effectiveness of your detection equipment must be done regularly, but how often depends on your site’s unique requirements and risks. Customer specifications, operational capabilities, and contingency plans for system failures are factors that should be taken into account. Effective systems go beyond testing frequency by establishing clear corrective actions in case of failures. This could include isolating and reinspecting affected products to safeguard food integrity.
Finally, when equipment detects or removes foreign material, a deeper investigation should follow. Identifying the source of the contamination and understanding trends from rejected materials can help you make informed decisions about preventive actions. Proactive management not only reduces the risk of recurrence but also enhances the overall robustness of your foreign-body control programme.

Filters and Sieves: Practical Control for Foreign Bodies
The use of filters and sieves in food production plays an important role in controlling foreign body contamination. These devices must be designed and selected with care, ensuring they have a specified mesh size or gauge capable of providing the maximum practical protection for the products being processed. Choosing the appropriate specification is key to ensuring effective protection while maintaining operational efficiency.
Maintenance and Inspection Requirements
Regular inspection or testing of filters and sieves is essential for maintaining their integrity. The frequency of these checks should be documented and based on the level of risk associated with the production process. This proactive approach helps to detect any damage that might compromise product safety.
If defective filters or sieves are found, it’s not just a matter of replacing them. You need to record the incident and investigate whether any contamination may have occurred. Taking swift, appropriate action following the discovery of damage is essential to maintaining product integrity and protecting consumer safety.
These steps are vital to ensuring that your foreign body control measures remain robust and effective, contributing to the overall safety and quality of your food products.

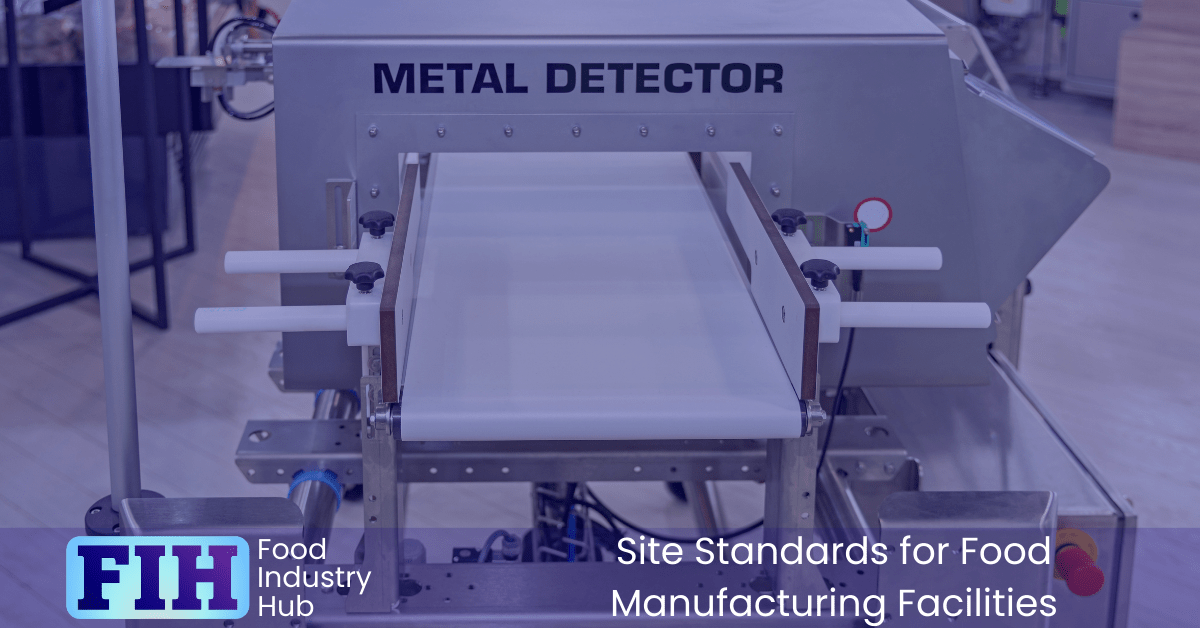
Metal Detectors and X-ray Equipment
The Role of Detection Equipment in Food Safety
Effective use of metal detectors and X-ray equipment is essential for identifying and removing physical contaminants from food products. These tools act as critical control points, reducing the risk of foreign body contamination, which can pose serious food safety risks and damage consumer trust. Ensuring the right detection system is in place and managed effectively will safeguard your products and reputation.
Justifying Absence of Metal Detection
If your site chooses not to use metal detection, you must be prepared to justify this decision through a documented risk assessment. It’s not enough to simply deem it unnecessary — you need evidence that an alternative method provides equivalent or superior protection. Options might include using X-ray equipment, fine sieves, or filtration methods. This rationale must withstand scrutiny from auditors and meet regulatory expectations.
System Requirements for Detection
When incorporating metal detectors or X-ray systems, it’s vital to select the appropriate features based on your product and operational requirements:
- Automatic Rejection Devices: These are invaluable for continuous in-line systems, allowing for swift and secure removal of contaminated products.
- Belt Stop Alarms: For large or bulky packs, where automatic rejection isn’t feasible, an alarm-triggered belt stop can effectively alert operators.
- In-line Detection Points: Systems that identify where contamination occurs enable targeted corrective actions and efficient product segregation.
These design choices not only ensure effective contamination management but also streamline operations by minimising unnecessary production disruptions.
Operational and Testing Procedures
Developing robust procedures for operating and testing your detection equipment is non-negotiable. At a minimum, your protocols should address:
- Responsibility Assignments: Clearly define who is responsible for conducting tests and maintenance.
- Operating Effectiveness: Validate the sensitivity settings regularly and document any adjustments for specific product types.
- Inspection Frequencies: Determine testing intervals based on the risk profile of your operations and products.
- Record Keeping: Maintain detailed logs of test results to demonstrate due diligence and facilitate trend analysis.
Taking these steps will help ensure that your detection systems remain effective and compliant, protecting both your business and your customers.
Metal Detector and X-ray Testing Procedures
Establishing Effective Testing Practices
Thorough and accurate testing of both metal detectors and X-ray equipment is essential to ensuring they remain effective in detecting foreign-body contaminants. These systems are only as reliable as the procedures that govern their use, so getting the testing protocols right is vital.
Metal Detector Testing
To ensure metal detectors perform optimally, your testing procedures should cover:
- Use of Test Pieces: Test pieces should incorporate spheres of specific sizes and materials (such as ferrous, stainless steel, or non-ferrous metals) based on the assessed risk.
- Separate Test Runs: Tests for each material type should be carried out independently to confirm accurate detection.
- Detection and Rejection Validation: Check that both the detection and rejection mechanisms operate correctly under typical operating conditions and speeds.
- Failsafe Systems: Ensure that failsafe features designed to manage detection failures are functioning as intended.
For in-line metal detectors, it’s essential to test as close as possible to the least sensitive area of the aperture. Placement accuracy for the test piece and the rejection timing validation are also critical elements of your testing process.
X-ray Equipment Testing
Similar principles apply to X-ray equipment testing, with particular attention to:
- Test Material Selection: Choose spheres of suitable material sizes based on the specific contaminants you’re targeting.
- Independent Test Runs: Run separate tests to validate the equipment’s ability to detect different types of foreign bodies.
- Operational Verification: Confirm that both detection and rejection mechanisms are fully functional during routine operations.
- Failsafe Checks: Validate that system failsafe mechanisms are correctly configured and operational.
When testing in-line systems, always ensure test pieces are placed at the least sensitive areas, such as near the X-ray source or detector. Timing adjustments for rejection systems and periodic tests during start-up and end-of-production periods are also critical components of maintaining system accuracy.
Practical Insights
- Always document testing results meticulously, not just for compliance but for ongoing performance improvement.
- Be proactive in identifying trends from test failures, using them to enhance equipment setup or refine operating conditions.
- Ensure staff are fully trained to perform tests correctly and interpret results accurately.
By maintaining a rigorous testing protocol, you’ll strengthen your site’s defences against physical contamination, reinforcing your commitment to food safety and quality assurance.
Magnet and Optical Sorting Equipment Procedures
Documenting Magnet Usage and Integrity Checks
Magnets play an important role in the detection and removal of ferrous foreign bodies. Ensuring they operate effectively requires a structured approach:
- Document Type, Location, and Strength: Record all relevant details, including the specific type of magnets used, their positioning on the production line, and the required strength to maintain food safety protection.
- Establish Inspection and Maintenance Procedures: Implement scheduled checks to assess the magnets’ strength and integrity. Include visual inspections for cleanliness and physical damage.
- Final Product Testing with Magnets: Integrate magnet use into product safety testing routines where appropriate to safeguard against contamination.
- Record-Keeping: Maintain comprehensive records of inspections, cleaning routines, and testing results. These records will support compliance verification and help track equipment performance over time.
A failure to maintain magnet effectiveness could increase the risk of physical contamination, so regular checks and prompt maintenance are essential safeguards.
Ensuring the Effectiveness of Optical Sorting Equipment
Optical sorting equipment provides a modern, technology-driven method for detecting and removing a variety of contaminants. Maintaining its effectiveness requires adherence to the following best practices:
- Follow Manufacturer Guidelines: Conduct equipment checks and maintenance in strict accordance with the manufacturer’s specifications. This includes calibration and operational checks to ensure the machine continues to detect and reject defective products accurately.
- Document Procedures and Checks: Keep detailed records of equipment inspections, fault reports, and adjustments. Well-maintained documentation demonstrates due diligence and supports system validation efforts.
By combining strong documentation practices and regular maintenance, you’ll ensure your site’s detection equipment continues to protect food safety while reducing contamination risks.

Container Cleanliness for Rigid Packaging
Minimising Contamination from Packaging Containers
Packaging materials, such as glass jars, cans, and other pre-formed rigid containers, can be a source of physical contamination if not properly controlled. Risk assessments should guide the development of procedures to reduce contamination risks.
- Preventive Measures: Implement strategies such as the use of covered conveyors, which shield open containers from potential contaminants. Container inversion processes combined with water or air jets can be effective in removing foreign bodies before containers are filled.
- Tailored Solutions: Your choice of cleaning method should align with the type of container used and the identified contamination risks. For instance, glass containers may require gentler yet thorough cleaning due to their fragility compared to metal cans.
By investing in robust procedures, you significantly reduce the risk of contaminants entering your final product through the packaging itself.
Verifying the Effectiveness of Container-Cleaning Systems
It’s critical to monitor the efficiency of your container-cleaning systems to maintain food safety standards throughout production.
- Routine Checks and Records: Ensure each production run includes documented checks on cleaning equipment effectiveness. These records support traceability and compliance efforts.
- Detection and Rejection Tests: Where rejection systems are in place to handle dirty or damaged containers, validate their functionality by regularly testing their ability to both detect and reject faulty containers.
Effective control of container cleanliness not only protects your product but also strengthens customer confidence in your commitment to delivering safe and high-quality food products.

Other Foreign-Body Detection and Removal Equipment
Ensuring Proper Operation and Maintenance
Foreign-body detection and removal equipment plays a critical role in protecting food safety, particularly when handling bulk materials or specialised processes. These systems might include technologies such as gravity separation, fluid bed technology, and aspirators.
- Following Manufacturer Recommendations: Each piece of equipment is designed with specific operational and maintenance protocols in mind. Ensure you’re adhering to the manufacturer’s guidance for checks and servicing. This not only optimises performance but also extends the lifespan of the equipment.
The Importance of Documenting Checks
- Comprehensive Records: Maintaining detailed documentation of all equipment checks supports your due diligence efforts. This demonstrates your proactive approach to contamination control and strengthens your food safety management system.
- Traceability and Continuous Improvement: These records allow you to track performance trends and identify areas for process improvement or additional training needs.
By staying vigilant and maintaining your detection and removal systems according to best practices, you significantly reduce the risk of foreign-body contamination in your products.
Housekeeping and Hygiene
Cleanliness as a Cornerstone of Food Safety
A clean and hygienic environment is fundamental to ensuring safe food production. Effective housekeeping practices reduce the risk of product contamination and help maintain compliance with regulatory and customer requirements.
Documented Cleaning Procedures
Developing and maintaining detailed cleaning and disinfection procedures for all areas and equipment is essential. You’ll need to ensure these procedures clearly outline:
- Roles and Responsibilities: Assign specific tasks to individuals to avoid confusion and ensure accountability.
- Cleaning Frequencies and Methods: Define when and how cleaning should be conducted, including dismantling equipment where necessary.
- Verification Steps: Implement sign-off checks and records to confirm cleaning has been completed correctly.
- Chemical Usage: Specify approved cleaning agents, concentrations, and handling requirements to avoid any unintended contamination.
By documenting these steps, you not only demonstrate due diligence but also create a reference point for training new staff and standardising practices across your site.
Defining Cleaning Performance Standards
To manage the risks associated with different types of contamination (microbiological, allergen, or foreign body), establish clear criteria for what constitutes acceptable and unacceptable cleaning results. Testing methods might include visual inspection, microbiological testing, or ATP swabbing depending on your operational risks.
- Corrective Actions: When cleaning outcomes fall short of acceptable levels, prompt corrective action is necessary to restore hygiene standards.
- Validation and Verification: Periodically validate that your cleaning procedures are effective and maintain records for traceability.
Scheduling and Resource Allocation
Effective cleaning requires thoughtful planning to minimise production disruptions. This may include scheduling non-production periods for dismantling large equipment and ensuring skilled personnel are available.
Pre-Production Cleaning Checks
Before starting production, perform thorough checks to confirm that equipment is clean and ready for use. Analytical and microbiological tests provide additional confidence where risks are high.
Equipment Design and Storage
Hygienically designed equipment is easier to clean and less likely to harbour contaminants. Ensure tools are properly stored, colour-coded where applicable, and protected from potential contamination.
By maintaining meticulous hygiene standards, you’re not just protecting product safety—you’re building a culture that prioritises quality and operational excellence.

Cleaning in Place (CIP) Systems
Ensuring Effective Design and Operation
The design and construction of your CIP system are foundational to its effectiveness. Validation is essential to confirm the system’s correct operation and compliance with hygiene requirements. You need to ensure:
- Accurate System Documentation: Maintain an up-to-date schematic diagram that reflects the actual layout of your CIP system.
- Risk Assessments for Rinse Solutions: Evaluate the potential risks when rinse solutions are recovered and reused, particularly the chances of cross-contamination.
- Revalidation of the System: Periodically revalidate the system based on risk assessments or after significant alterations to maintain its effectiveness.
By addressing these considerations, you protect your products from contamination risks associated with CIP inefficiencies.
Defining Performance Limits
Clear limits for key process parameters are critical to achieving effective cleaning. These should address:
- Cycle Times: Specify appropriate cleaning durations for different stages of the CIP process.
- Detergent Concentrations and Temperatures: Maintain defined levels for optimum cleaning efficiency without compromising safety.
- Flow Rates and Pressure Levels: Ensure sufficient coverage and agitation for effective cleaning.
Validation of these parameters is necessary to ensure consistent and repeatable results.
Maintenance of CIP Equipment
Regular maintenance by trained staff helps you keep the system in peak condition. Key maintenance activities should include:
- Routine checks on detergent concentrations and tank solutions.
- Filter cleaning and inspection at defined frequencies.
- Proper storage and inspection of hoses to prevent contamination.
This proactive approach to maintenance reduces the risk of cleaning failures.
Monitoring and Continuous Improvement
Monitor CIP facilities at defined intervals to ensure they meet operational requirements. This involves:
- Process Parameter Monitoring: Ensure parameters remain within defined limits.
- Operational Checks: Verify that all components are functioning as intended, including valves and spray balls.
- Effective Completion Checks: Ensure full drainage and rinsing after cleaning cycles.
When monitoring identifies deviations, it’s critical to act swiftly. Define clear corrective procedures to manage such instances, ensuring that product safety remains uncompromised.
By investing in the design, validation, and maintenance of your CIP system, you lay the groundwork for a robust and effective cleaning process that supports food safety and operational efficiency.
Environmental Monitoring
Designing a Risk-Based Monitoring Programme
An effective environmental monitoring programme (EMP) is critical for identifying risks from pathogens and spoilage organisms in your production areas. Your design process should be comprehensive and risk-based, incorporating:
- Sampling Procedures and Locations: Strategically identify and define sample points that are most likely to highlight potential contamination risks.
- Frequency of Testing: Base test frequency on the risk level of each production zone. Higher-risk areas, such as those handling ready-to-eat products, will require more frequent testing.
- Target Organisms and Test Methods: Select pathogens, spoilage organisms, or indicators relevant to your products and processing environment. Consider diverse methods, such as settle plates, swabs, and rapid testing, depending on your needs.
- Result Evaluation and Documentation: Maintain clear records of test results, trends, and interpretations to support ongoing risk management efforts.
A well-documented and thoughtfully executed programme is your foundation for proactive environmental control.
Setting and Managing Control Limits
Defining control and action limits ensures your EMP delivers actionable insights. These limits must align with product risk levels and legal or industry requirements.
When test results breach control limits or reveal concerning trends, immediate corrective actions should be documented and implemented. By addressing trends toward non-conformance, you protect against product contamination and maintain compliance.
Reviewing and Improving Your Programme
Environmental monitoring is not static; it must evolve to reflect changes in production, scientific advancements, and regulatory expectations. Reviews should occur annually or in response to:
- Operational Changes: Updates to process flows, equipment layouts, or factory conditions.
- Scientific Developments: Emergence of new pathogens or risk information.
- Programme Failures: Instances where risks missed by your programme are identified.
- Product Failures: Positive results from finished product tests or repeated contamination events.
- Consistently Negative Results: Sites with no positive findings should reassess their testing strategies to ensure adequate coverage and relevance.
By regularly reviewing your environmental monitoring programme and adapting it as necessary, you strengthen your site’s defences against contamination risks and reinforce your commitment to food safety.

Waste and Waste Disposal
Legal Compliance and Record Management
Effective waste management starts with adherence to legal requirements. If waste removal requires licensing, you must engage licensed contractors and maintain comprehensive records of waste collection and disposal. These records should always be available for audits, ensuring traceability and accountability.
Managing Waste Facilities
Both internal and external waste collection areas need to be carefully managed to minimise contamination risks and pest attraction. Proper management includes:
- Clear Identification: Labelling waste collection points clearly to avoid errors in disposal.
- Ease of Use and Cleaning: Designing containers to facilitate effective waste disposal and cleaning.
- Maintenance and Hygiene: Keeping containers clean and disinfected where necessary to prevent contamination.
- Frequent Emptying: Scheduling waste removal at intervals that prevent overflow and accumulation.
- Sealed Storage: Ensuring external containers are covered, or doors are kept closed to prevent pest entry.
These practices protect your site from contamination risks and demonstrate a proactive approach to hygiene and safety.
Waste Removal from Open Product Areas
Particular care is necessary for waste in open product areas. Any waste handling process in these zones must be designed to protect product safety and maintain a clean production environment. This can involve frequent waste collection and clearly defined removal routes that avoid contamination hotspots.
Disposal of Unsafe or Substandard Products
If unsafe products or substandard trademarked materials need disposal, you must engage a secure specialist service provider. They must provide traceable records that detail the quantity of waste collected and confirm its destruction or disposal.
By managing waste disposal effectively, you not only comply with legal requirements but also protect your products, brand reputation, and operational hygiene standards.

Management of Surplus Food and Products for Animal Feed
Handling Surplus Customer-Branded Products
If your site handles surplus products bearing customer brand names, it’s important to comply with the customer’s specific disposal requirements. These brand names must be removed from any surplus products before they leave the factory unless explicit customer authorisation is given. This helps safeguard the customer’s brand reputation and prevents unauthorised resale or misuse of branded goods.
Sale or Donation of Non-Specification Products
In cases where products that don’t meet specifications are sold to staff or donated to charities, this should only occur with prior consent from the brand owner. You must also ensure that:
- All products, whether own-branded or customer-branded, are fit for human consumption.
- Products meet legal requirements.
- Full traceability of the products is maintained.
Having robust procedures for this ensures compliance with food safety standards and demonstrates responsible product handling.
Animal Feed Products
For by-products or downgraded surplus products designated for animal feed, stringent segregation practices are necessary. These products must be stored securely and protected from contamination. Additionally, compliance with relevant legislative requirements governing animal feed products must be maintained, ensuring their safety and legality.
By carefully managing surplus products, your site not only avoids unnecessary waste but also demonstrates compliance, traceability, and a responsible approach to product safety and sustainability.

Pest Management
Preventing and Managing Infestations
Effective pest management is crucial for maintaining food safety and protecting raw materials and packaging. If pests are detected, they must not pose a contamination risk. Any sign of infestation should be documented and prompt action taken to eliminate or control the issue, ensuring no risk to products. This requires a proactive approach with a preventive pest management programme supported by detailed records and a clear action plan.
Contracted vs. In-House Pest Management
If you choose to contract a professional pest management service, ensure the contractor is competent and regularly inspects the site. The frequency of inspections should be determined through risk assessment and adjusted whenever:
- There are changes to buildings or production processes impacting pest management.
- A significant pest issue arises.
Clearly define the scope of work for external contractors to align with regulatory requirements. Conversely, if managing pests in-house, your team must be fully trained, competent, and have the resources to:
- Select and safely use pest control chemicals and proofing methods.
- Respond swiftly to any infestations.
- Comply with legal requirements for training or registration.
- Access specialist technical support when needed.
Documentation and Record Keeping
Maintaining comprehensive records is essential for effective pest management. These should include:
- A detailed site map indicating pest control devices and monitoring stations.
- Responsibilities of the site and the contractor.
- Specifications for pest control devices and instructions for their use.
- Records of all pest control activities, including treatments and inspections.
Records can be maintained on paper or electronically, but they must be accurate, up-to-date, and readily accessible for review.
Bait Stations and Control Devices
Rodent monitoring and control devices, including bait stations, must be strategically positioned to prevent contamination risks. In production or storage areas, toxic baits are generally prohibited, except when actively treating an infestation. Ensure that these are securely placed, and document any missing bait stations, investigating the cause.
Implementing a robust pest management programme is vital to safeguarding food safety and maintaining compliance with legal requirements.
Insect-Killing Devices and Bird Prevention
Proper placement and operation of insect-killing devices, such as EFKs, are essential to effective pest management. These devices should be strategically located to minimise contamination risks. If there’s a risk of insects being expelled and contaminating products, consider using alternative systems. Additionally, preventive measures must be in place to stop birds from entering buildings or roosting near loading and unloading areas, as they pose significant contamination risks.
Responding to Pest Activity
If signs of infestation or pest activity are detected, immediate action is necessary to identify at-risk products and minimise contamination risks. This involves implementing non-conforming product procedures and swiftly addressing any affected areas. Rapid and effective response protocols help prevent minor issues from escalating into significant contamination events.
Record-Keeping and Accountability
Maintaining detailed records of pest management activities, including inspections, pest-proofing measures, hygiene recommendations, and corrective actions, is crucial for accountability and traceability. It’s the site’s responsibility to ensure timely implementation of all recommendations made by contractors or in-house experts. Proper documentation not only supports food safety but also aids in regulatory compliance and audit readiness.
Annual Pest Management Assessment
Conducting an in-depth, documented pest management assessment at least once a year is essential to evaluating and enhancing the effectiveness of pest control measures. This should be carried out by a pest management expert who will:
- Inspect the site, equipment, and facilities for signs of pest activity.
- Review and assess the current pest management strategies, recommending changes where necessary.
Scheduling these assessments strategically allows for thorough inspection of equipment, especially where stored product insect infestations could occur. This proactive approach ensures any potential risks are identified and managed before they impact food safety.
Trend Analysis and Continuous Improvement
Regular analysis of pest management inspection results is key to identifying trends and improving pest control strategies. At a minimum, this analysis should occur:
- Annually, as part of routine monitoring.
- Whenever an infestation is detected.
Data from trapping and monitoring devices should be analysed to pinpoint problem areas. This trend analysis should then inform adjustments to pest management procedures, ensuring continuous improvement and enhanced effectiveness.
Staff Training and Awareness
Your pest management programme is only as effective as the people implementing it. Staff should be trained to recognise signs of pest activity and understand the urgency of reporting any evidence to a designated manager. This requires ongoing education and reinforcement to maintain vigilance and a proactive food safety culture.
An effective pest management strategy hinges on a comprehensive approach encompassing preventive measures, rapid response to issues, thorough record-keeping, and continuous improvement through regular assessment and staff engagement. This not only safeguards food safety but also ensures compliance with regulatory standards.

Storage Facilities
Ensuring Product Safety and Quality During Storage
To maintain product safety and quality during storage, robust procedures based on risk assessment are essential. These procedures should be well understood by relevant staff and implemented consistently. Key considerations include:
- Managing the transfer of chilled and frozen products between temperature-controlled areas to maintain product integrity.
- Segregating products to prevent cross-contamination, particularly where microbiological or allergenic risks exist, or where taint uptake could occur.
- Storing materials off the floor and away from walls to facilitate cleaning and pest control.
- Using specific handling or stacking requirements to avoid product damage during storage.
Implementing these controls helps mitigate risks associated with storage, safeguarding both product quality and safety.
Protecting Packaging from Contamination
Packaging materials should be stored separately from raw materials and finished products to prevent cross-contamination. This is particularly important for part-used packaging, which must be clearly identified and protected before returning to storage. Proper segregation and identification are crucial for maintaining traceability and ensuring product safety.
Temperature Control for Sensitive Products
Where temperature control is critical, such as for raw materials, semi-finished products, or final products, the storage area must consistently maintain the specified temperature. This requires:
- Installing temperature recording equipment with alarms to monitor storage conditions continuously.
- Implementing a system of recorded manual temperature checks, typically at least every four hours, to verify compliance with defined safety, legality, or quality limits.
Prompt intervention protocols should be in place to address any deviations, preventing product safety or quality issues.
Controlled Atmosphere Storage
If controlled atmosphere storage is required, the specific conditions must be clearly defined and effectively monitored. This involves maintaining detailed records of storage conditions to ensure consistency and traceability. Proper management of controlled atmospheres is essential for extending product shelf life and maintaining quality.
Outdoor Storage Considerations
In cases where outdoor storage is necessary, stringent measures must be in place to protect items from contamination and deterioration. This includes thorough checks for suitability before bringing items into the factory. Outdoor storage poses unique challenges, so regular inspections are critical to ensuring product safety.
Stock Rotation and Material Use
An effective stock rotation system should be implemented to ensure raw materials, intermediate products, and finished products are used in the correct order. This not only prevents the use of expired materials but also supports the traceability of batches, helping to manage product recalls efficiently if necessary.
Proper stock rotation is particularly important for materials with limited shelf life or specific usage requirements. This proactive approach helps maintain product quality and reduces waste.
Effective management of storage facilities requires a comprehensive approach that addresses risk assessment, contamination prevention, temperature control, and stock rotation. By implementing these best practices, you can ensure product safety and quality throughout the storage process.

Dispatch and Transport
Ensuring Product Safety and Quality During Loading and Transportation
Effective procedures must be in place to maintain product safety and quality throughout loading and transportation. This involves:
- Controlling the temperature of loading dock areas and vehicles to preserve product integrity.
- Using covered bays for vehicle loading or unloading to protect products from environmental contamination.
- Securing loads on pallets to prevent movement during transit, minimising the risk of damage.
- Conducting thorough inspections of loads before dispatch to identify any potential risks.
These measures help ensure that products are protected from contamination and damage during transport, maintaining their safety, quality, and legality.
Vehicle and Container Requirements for Transport
All vehicles and containers used for transporting raw materials and finished products must be fit for purpose. This includes ensuring that they are:
- In a clean condition, free from contaminants that could compromise product safety.
- Free from strong odours that may cause taint issues.
- Structurally sound and suitable for preventing damage to products during transit.
- Equipped to maintain temperature requirements where necessary to ensure product quality.
Regular inspections and detailed records of these checks are crucial to verifying compliance and maintaining traceability.
Temperature Control During Transport
Where temperature control is critical, vehicles must maintain product temperatures within the specified range. This requires:
- Using data-logging devices that can be interrogated to confirm time and temperature conditions.
- Implementing systems to monitor and record the operation of refrigeration equipment at set frequencies.
These controls are essential for products that are temperature-sensitive, ensuring they arrive at their destination in optimal condition.
Maintenance and Cleaning of Transport Equipment
Maintenance systems and documented cleaning procedures must be established for all vehicles and equipment used for loading and unloading. Detailed records of cleaning and maintenance activities should be maintained to demonstrate compliance and ensure that equipment is safe and fit for use.
This reduces the risk of contamination and ensures the longevity and reliability of transport equipment.
Transport Security and Contingency Planning
Clear procedures are required to manage the security of products during transport, including:
- Restrictions on the use of mixed loads to prevent cross-contamination or security breaches.
- Security requirements for products during transit, particularly when vehicles are parked and unattended.
- Clear instructions for drivers in the event of vehicle breakdown, accidents, or failure of refrigeration systems, ensuring product safety and integrity are maintained.
Effective security measures protect against tampering, theft, and contamination risks during transportation.
Managing Third-Party Transport Contractors
If third-party contractors are used, a documented supplier approval procedure is necessary to ensure they comply with safety and quality standards. This approval can be based on:
- Certification to a recognised standard, such as the BRCGS Global Standard for Storage and Distribution or a GFSI-benchmarked standard.
- A comprehensive contract outlining terms and conditions, verified by a demonstrably competent person.
These measures ensure that third-party transport operations align with the company’s product safety and quality requirements.
Proper management of dispatch and transport activities is critical for safeguarding product safety, quality, and legality during distribution. By implementing these best practices, you can minimise risks and maintain consumer confidence.

In Summary
Maintaining high site standards in your food manufacturing facility is not just about compliance—it’s about ensuring the safety, quality, and integrity of your products at every stage of handling, storage, and transport. From robust pest management to carefully controlled storage conditions and secure dispatch processes, each element plays a vital role in protecting food from contamination and maintaining consumer trust. By implementing and continuously improving these standards, you create a safer, more efficient operation that meets regulatory expectations and strengthens your reputation in the industry.

Further Resources
Food Industry Hub serves the food industry with a range of digital resources for the benefit of both commercial food manufacturers and food industry professionals.
For food manufacturers, we offer integrated management systems that give every user a direct interface with your QMS.
For food industry professionals, we provide an extensive signposting service in addition to informational content we hope you’ll find useful as you face new professional challenges. We have very ambitious plans to expand the range of services offered, and currently present informational content on management, safety and quality, food safety and quality culture, and professional success.














